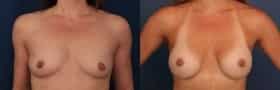
Board-certified plastic surgeon Dr. Ronald Schuster has been helping women achieve elegant, natural looking breasts for nearly 30 years. If you are unhappy with the size and shape of your breasts, breast augmentation may be the ideal solution to attain your aesthetic goals.
To discuss breast augmentation and your candidacy for this popular procedure, please call 410-902-9800 today to schedule a personal consultation with Dr. Schuster. In addition to serving the Baltimore, Owings Mills and Towson areas of Maryland, we frequently help women from throughout the country look and feel their best. Virtual consultations are available for out-of-town patients.
On this page, we discuss the following topics:
- What Is Breast Augmentation?
- Top 5 Reasons Women Get Breast Implants
- Different Types of Breast Implants
- Breast Implant Shapes
- Breast Implant Sizing
- Breast Implant Textures
- Breast Implant Profiles
- Breast Augmentation Recovery
- Breast Implant Scars
- Breast Augmentation with a Breast Lift
- Will I Be Able to Breastfeed if I Get Breast Implants?
- Are Breast Implants Safe?
- Why Choose Dr. Ronald Schuster for Your Breast Augmentation?
- How Much Does Breast Augmentation Cost?
- Will Insurance Pay for my Breast Augmentation?
- Schedule Your Breast Augmentation Consultation Today
What Is Breast Augmentation?
Breast augmentation, sometimes referred to as a “boob job” by patients, is a surgical breast procedure that enhances the size and shape of the breasts with artificial implants or the patient’s own fat. Breast augmentation appeals to women from all walks of life that have naturally small, asymmetrical or disproportionate breasts as well as women who have lost breast volume or symmetry because of pregnancy, breastfeeding or the effects of the aging process.
Many women feel an innate connection between their breasts and their sense of femininity and confidence. Are you one of them? Perhaps you’ve always had small breasts, or you noticed your breasts lost volume after nursing your babies. You might feel less feminine or less self-confident because of the appearance of your flat chest. Breast augmentation can help.
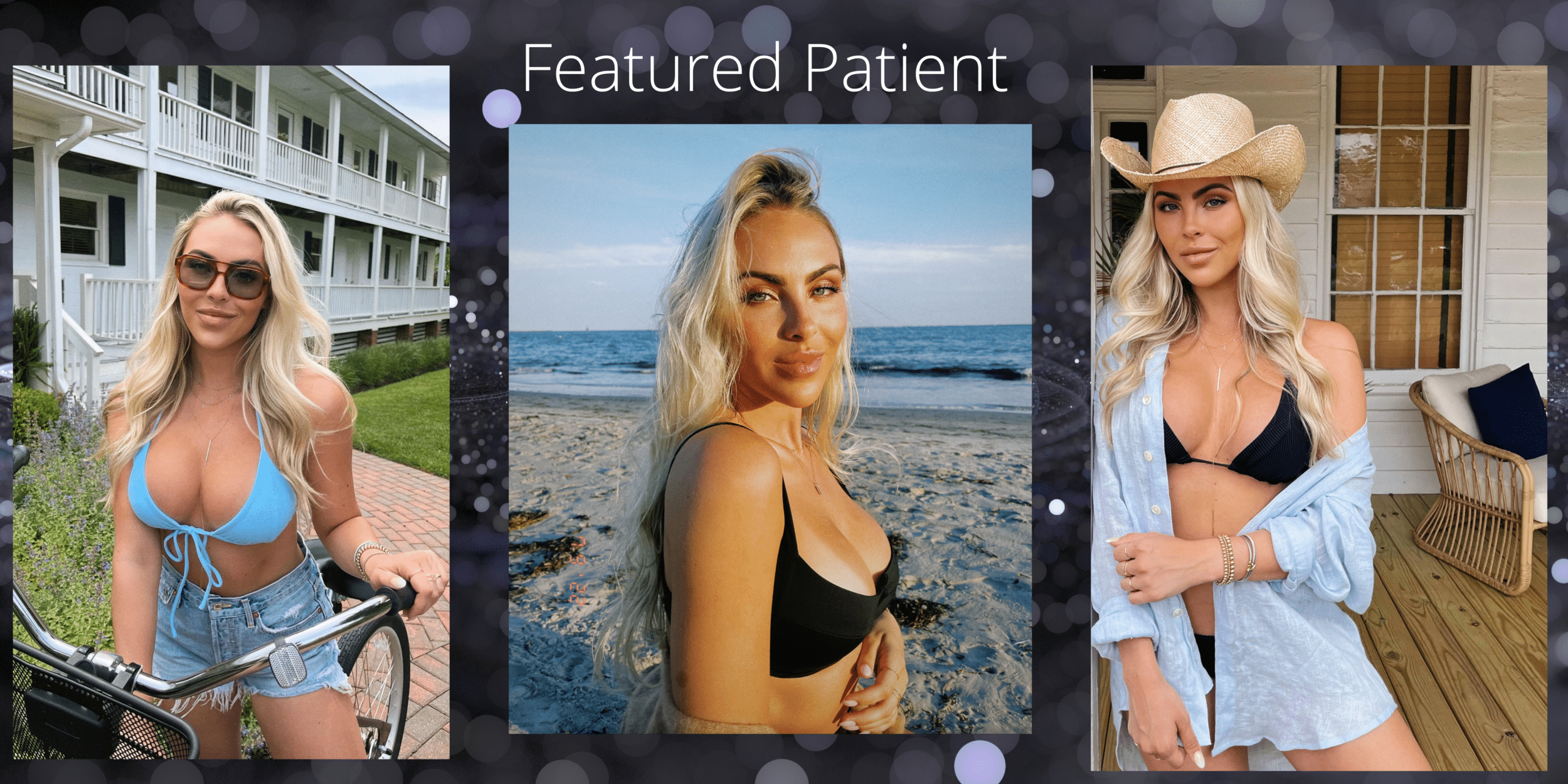
Our featured breast augmentation patient is age 24. She wanted an augmentation her entire life. She received Sientra Gel moderate plus implants 410cc and went from a 34A to a 34D. She is thrilled with her results and new found confidence.
Top 5 Reasons Women Get Breast Implants
- To improve confidence
- To feel sexier and younger
- To increase their wardrobe options
- To improve the symmetry of the breasts
- To restore their breast size and shape after pregnancy/breastfeeding
Different Types of Breast Implants
Implants come in a variety of sizes, shapes (round and teardrop) and profiles (moderate, moderate-high, high, etc.). The type, size, and shape used depends on the woman’s breasts, skin, chest size, and breast enlargement goals.
- Saline Breast Implants: A saline breast implant is filled with a sterile saltwater solution encased in a silicone shell. To place saline implants, Dr. Schuster normally inserts the empty saline implant shell into the breast and then fills it to the desired volume with the saline solution. One of the advantages to saline implants is that in the rare event of a leak or rupture, the body safely absorbs the saltwater solution and the breast is noticeably flattering almost immediately.
- Silicone Breast Implants: Silicone breast implants are filled with silicone gel. They are usually shipped from the manufacturer pre- filled and require a slightly larger incision to place than saline implants. Although every woman is different, most believe that silicone implants look and feel more natural than saline implants. However, one drawback to silicone implants is that in the rare event of a leak or rupture, the silicone tends to stay in or around the breast pocket and it may not be noticeable that a complication has occurred.
- “Gummy Bear” Breast Implants: Gummy bear implants are form-stable silicone breast implants. Like their namesake, gummy bear implants retain their shape even when cut in half.
With the use of any implant, there are many issues to be discussed. These include issues about how implants are made, how long they last, what happens when they wear out and how they might affect the body. The breast implant selection process also has to take into consideration the patient’s goals as well as her anatomy — the width of her natural breast, softness of the skin, amount of subcutaneous fat, asymmetries and any other chest wall irregularities.

Discussion of these important issues, as well as many others, is beyond the scope of this website. A full hour is dedicated to discussing these options during your breast augmentation consultation. Call our Baltimore, Maryland-area office to book an appointment or to get more information from our breast augmentation surgeon.
Breast Implant Shapes
You probably already know that breast implants come in different sizes. You also probably know by now that breast implants come with different fillings. But what you may not have realized is that breast implants come in different shapes as well. It’s true. Our Baltimore cosmetic surgeon, Dr. Ronald Schuster, has helped many patients enhance their body contours through breast augmentation surgery. To help keep prospective patients informed, we figured it would be a good idea to go over the different shapes of breast implants: round and teardrop-shaped.
- Round Breast Implants: Round breast implants are the most common breast implants used in terms of shape. These kinds of breast implants are shaped like a ball that has been partially flattened. (It might be best to think of them as being slightly domed.) These round breast implants tend to provide a good amount of lift and projection for the breasts, and are ideal for enhancing fullness and enhancing cleavage.
- Teardrop-Shaped Breast Implants: Teardrop-shaped breast implants are the other type of breast implant shape used in Baltimore breast augmentation surgery. Teardrop-shaped breast implants have a gradual slope to them, and because of this, they tend to look more like natural breasts than round breast implants. One concern with teardrop-shaped breast implants, however, is implant rotation can cause a distorted appearance of the breast.
The shape of the breast implants that are best for you will be determined by your needs as a patient and your goals for the breast augmentation surgery. Dr. Schuster will help you make the best decision based on your needs and your body type.
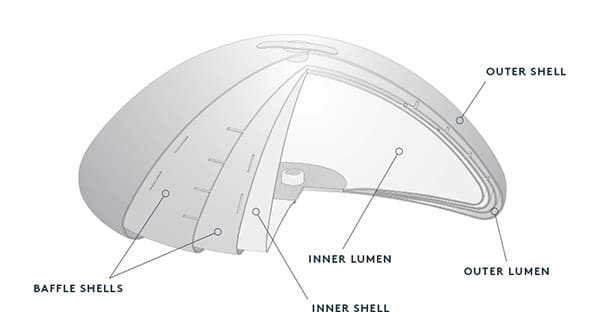
Breast Implant Sizing
The breast implant size that is most appropriate for your case depends on factors like your desired outcomes, your body type and the amount of natural breast tissue you have. The implant size that works best for a tall, broad-shouldered patient doesn’t look the same on a petite woman, and vice versa.
Breast Implant Textures
Breast implants come in two different textures:
- Smooth implants have a smooth and sleek surface and can move around in the breast pocket.
- Textured implants have a slightly rough surface to adhere to the surrounding tissue in the breast pocket, making them less likely to move around in the pocket.
Breast Implant Profiles
The profile of a breast implant refers to how much of the implant projects forward from the chest wall when a woman is standing. High-profile implants project furthest from the chest to create the fullest and most rounded results, as compared to a low- or moderate-profile implant that offers less projection.
Top 5 Reasons Women Get Breast Implants
Your reasons for seeking breast augmentation are unique! Talk to Dr. Schuster to make sure those expectations align with what the procedure can achieve. Some of the reasons people express interest include:
- To improve confidence. Larger breasts may provide that extra “oomph” that makes you feel like the best version of yourself.
- To feel sexier. Surveys conducted by the American Society of Plastic Surgeons show that many women report increased levels of sexual satisfaction after breast augmentation.
- To increase their wardrobe options. While you can of course wear anything you want regardless of the size of your breasts, you may find that a broader range of clothing is more flattering after breast augmentation. Larger breasts may allow you to fill out dresses and blouses that you didn’t fill out before.
- To improve the symmetry of the breasts. Breasts are sisters, not twins. All women experience some degree of asymmetry between the breasts, some more than others. While no surgery can completely eliminate this asymmetry, breast augmentation can significantly improve it.
- To restore breast size and shape after pregnancy and breastfeeding. Pregnancy and breastfeeding tend to change the appearance of your breasts. Breast augmentation can provide enhancement that restores the shape of your breasts. It may be possible to combine the placement of breast implants with a breast lift, if you are also experiencing sagging breasts.
Who Is A Candidate For Breast Augmentation?
In general, good candidates for breast augmentation:
- Want larger breasts
- Are in generally good overall health
- Have realistic expectations of what the procedure can achieve
- Are choosing breast augmentation for themselves, not to fulfill someone else’s expectations or please someone else
- Are prepared to follow pre-operative and post-operative surgical instructions
First, Dr. Schuster will make sure that breast augmentation is the right procedure for the physical outcomes you want to see. Then he will make sure that you meet all the medical requirements for undergoing a surgical procedure.
How Do I Prepare For Breast Augmentation Surgery?
Some of the steps in surgical preparation include:
- Get blood work completed
- Quit smoking, if you smoke
- Eliminate alcohol in the 2 weeks beforehand
- Stop taking ibuprofen, aspirin, and certain herbal supplements as instructed by Dr. Schuster
- Schedule time off from work
- Arrange for someone to drive you home from surgery
- Arrange for someone to stay with you for 24 hours after surgery
- Arrange for help around the house (i.e. childcare, pet care, errands)
- Prepare an area to rest and recover (i.e. with pillows, water, television, books, movies, etc.)
You will be asked to complete these steps in the 1-2 weeks leading up to breast augmentation. We provide complete and detailed instructions for your reference.
How Can I Maintain Results For My Breast Augmentation?
You don’t need to do much to enjoy your results for years and years to come. Here are a few ways you can make sure your breasts look as perky as possible:
- Follow all recovery guidelines. Reduce the risk of complications and poor results by following all recovery and aftercare instructions.
- Practice healthy skincare habits. Sun damage, excessive alcohol consumption, and smoking tend to have a detrimental effect on the skin all over your body, including your breasts. Care for your skin to keep it hydrated, protected, and nourished.
- Avoid weight fluctuations. Gaining and losing a lot of weight, especially repeatedly, may cause the skin of your breasts to stretch out. This can have a poor effect on your results, as your breasts might look droopy. Maintaining a stable body weight will help preserve your results.
Will I Need Future Surgeries For My Breasts After An Augmentation?
You may need a procedure to revise or replace your breast implants at some point down the road. There isn’t a set timeline in which this happens. Instead, some women choose to replace their breast implants to modify the size or type, and some people have a revisional procedure after the passage of years causes the breast implants to leak, deflate, or have some other issue.
Combining an Augmentation with Other Procedures
Yes, a breast augmentation can often be combined with other cosmetic procedures. One common combination is an augmentation-breast lift, in which sagging breasts are lifted with mastopexy and enlarged with breast implants. It may be possible to combine your breast augmentation with certain facial and body procedures, although you will need to discuss the possibility with Dr. Schuster. It may be better to separate the procedures into different appointments.
How Is Breast Augmentation Recovery?
Breast augmentation is performed as an outpatient procedure. Recovery is relatively easy; you can expect moderate swelling and discomfort for a few days after breast enlargement.
Plan to take at least one week off from work. While every patient heals at their own pace, most are able to return to full activity and sports about three weeks after breast augmentation surgery.
Breast Augmentation Before & After
Dr. Schuster will provide you with detailed pre and post-operative instructions to minimize the risk of complications and ensure a rewarding experience.
Breast Augmentation Consultation
During your one-hour private consultation at our Maryland office, Dr. Schuster will evaluate your breasts and overall anatomy and inquire about your goals of treatment. He will help you determine whether breast augmentation is a suitable solution.
If so, Dr. Schuster will help you select the size and shape of breast implants that are most likely to give you the results you want. He will also describe the various incision and placement techniques that can be used during surgery and discuss the pros and cons of each.
Breast Implant Scars
Breast implants are placed inside the chest through an incision created either around the edge of the areola (darkly pigmented skin surrounding the nipple), or in the crease of the breast where it meets the torso or in the armpit. The incisions create scars that fade nicely over time.

Featured Breast Augmentation Patient
Breast Augmentation with a Breast Lift
Dr. Schuster can recommend whether you would benefit from a breast lift at the time of breast augmentation. In general, you may need a lift if your breasts sit low on your chest and/or your nipples point downward.
Will I Be Able To Breastfeed If I Get Breast Implants?
Studies show that breast implants do not interfere with breastfeeding. Many women are able to successfully breastfeed after breast augmentation. If you plan to have children in the future, be sure to mention this to your breast augmentation surgeon during the consultation so he takes it into consideration when planning your surgery.
Are Breast Implants Safe?
Breast implants are among the most studied medical devices of all time. They are considered very safe when placed by an experienced, qualified breast augmentation surgeon like Dr. Schuster.
This is a very rare type of lymphoma that has been described to be possibly associated with breast implants. It generally presents itself late after surgery and behaves favorably. For more information, please review the following documents: BIA-ALCL Info, BIA-ALCL by the Numbers, BIA-ALCL FAQs, and BIA-ALCL Advisory Update.
Why Choose Dr. Ronald Schuster For Your Breast Augmentation?
Dr. Schuster is a board-certified plastic surgeon that hundreds of women have entrusted with their breast augmentations. Thanks to his world-class education, advanced training (including a prestigious fellowship program in plastic surgery) and many years of clinical experience in private practice, the breast augmentation surgeon is renowned for achieving natural-looking results that are perfectly suited to each individual patient’s treatment needs and goals.
As immediate Past Chief of the plastic surgery division at Northwest Hospital Center, Dr. Schuster is a highly sought-after authority on all types of breast surgery. His affiliations with distinguished professional organizations such as the American Society of Plastic Surgeons and the American Society for Aesthetic Plastic Surgery help keep him at the forefront of cosmetic medicine.
Breast Augmentation Patient Video Testimonials
Personal Trainer, Former Fitness Competitor
Former breast augmentation and tummy tuck patient talks about “getting her body back” after having triplets.*
Ruby, Former Patient
Former breast augmentation patient talks about her great experience with Dr. Schuster.*
Kara, Former Patient
Former patient shares how comfortable her office visit was and how well informed she was before breast surgery.*
Written Patient Testimonials
"Dr. Schuster was amazing! He gave me exactly what I wanted and didn’t try to push me in any direction with size. However, I was comfortable with knowing that he would not give me anything that didn’t fit my body. Dr. Schuster was thorough with his explanation of my options and took the time to answer all of my questions. I feel as though he genuinely invested in me and wanted me to feel better about my image."
How Much Does Breast Augmentation Cost?
The average cost of breast augmentation can range between $6,800 (saline implants) to $7,300 (gel/silicone implants) or $14,000 (breast augmentation with a lift). The exact cost of breast augmentation will depend on several factors, including:
- Type, shape and size of implant chosen
- Technique used to perform breast augmentation
- Whether the procedure is combined with breast lift surgery
- Necessary post-operative care
Dr. Schuster does not want breast augmentation cost at his cosmetic surgery practice to stand between you and the elegant, beautiful figure you desire. As one of our valued patients, you can be assured that we will work with you to make the cost of your breast augmentation as affordable as possible and explain all of your payment and financing options. Dr. Schuster and his team believe that breast enhancement of the highest standard should be available to anyone who wishes to refine and rejuvenate her appearance, regardless of her budget.
Will Insurance Pay For My Breast Augmentation?
Breast augmentation is considered elective and therefore not covered by insurance.
Schedule Your Breast Augmentation Consultation near Baltimore, MD Today
To book your confidential breast augmentation consultation, please contact our office online or call 410-902-9800 today. We serve Baltimore, Owings Mills and Towson, Maryland, and offer virtual consultations for out-of-town patients.